Home / Medical Supplies / ATROPINE SULFATE, PFS 0.1MG/ML10ML – #M286640
ATROPINE SULFATE, PFS 0.1MG/ML10ML – #M286640 $ 357.04
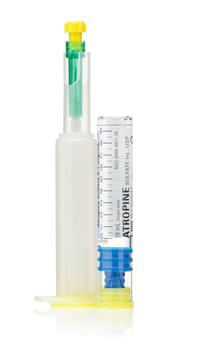
Antimuscarinic / Antispasmodic Agent Atropine Sulfate, Preservative Free 0.1 mg / mL Injection Prefilled Syringe 10 mL Adult
Medical License Required for Purchase.
Features
LifeShield™ Abboject™ glass syringe with an extended needle shroud plus a luer adapter
Includes 20 gauge x 1-1/2 inch needle
Atropine Sulfate Injection is a parenteral anticholinergic agent and muscarinic antagonist
The solution contains no bacteriostat, antimicrobial agent or added buffer (except for pH adjustment)
It is administered parenterally by subcutaneous, intramuscular or intravenous injection
Description
Manufacturer #
00409491134
Manufacturer
Hospira
Application
Antimuscarinic / Antispasmodic Agent
Container Type
Prefilled Syringe
Dosage Form
Injection
Generic Drug Code
18661
Generic Drug Name
Atropine Sulfate, Preservative Free
NDC Number
00409-4911-34
Storage Requirements
USP Controlled Room Temperature
Strength
0.1 mg / mL
UNSPSC Code
51151616
User
Adult
Volume
10 mL
Latex Free Indicator
Not Made with Natural Rubber Latex
McKesson #
286640
MSDS MSDS@cox.net
www.MSDSSafety.com
MSDS' best practices website is not a standard or regulation, and it creates no legal obligations, nor does it change any existing OSHA or other government standard or regulation. The guide is advisory in nature, informational in content, and is intended to assist employers in providing a safe and healthful workplace.







Reviews
There are no reviews yet.